Kinetic Model
Kinetic model is the general term containing the scheme (structure) of individual reaction steps in multi-step chemical reaction, Reaction typesReaction type is the elementary mechanism of one individual reaction step in multi-step chemical reaction. Reaction type f(Cr, Cp) describes dependence of the reaction rate for individual reaction step on the concentrations of reactant Cr and product Cp for this step.reaction types and kinetic parameters of these steps.
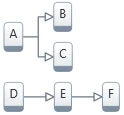
Scheme (structure) of kinetic model describes the connections of individual reaction steps in multi-step chemical reactions. Each reaction step can be individually connected to another reaction steps (consecutive, competitive, independent).
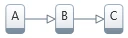
1. Single-step reaction:
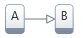
2. Two consecutive steps
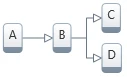
3. Three-step model with first consecutive step followed by two competitive steps:
4. Four-step model with two competitive steps and independent chain of two consecutive steps: