How To: Create a Simple Single Step Kinetic Model for DSC Data
Cis-trans isomerization of subcooled liquid Azobenzene
Introduction
Detailed description of this reaction and theoretical analysis can be found in the article N.Eckhardt, H.J. Flammersheim, H.K.Cammenga. CIS-TRANS isomerization of azobenzene in the molten state. Journal of Thermal Analysis, Vol.52, (1998) 177-185
In this "How To:", a single step kinetic model for DSC data will be created. These data have one exothermal peak due to isomerization of the sample. We will start by loading a sample data project included in Kinetics Neo, will then create a kinetics model consisting of one step.
Sample data:
- Data type: differential scanning calorimetry (DSC)
- Project file: Azobenc_Data.kinx
Load the Sample Data Project
1. Start the Kinetics Neo software.
Click on the blue "File" tab to open the application menu.
2. Open the Sample Data DSC project.
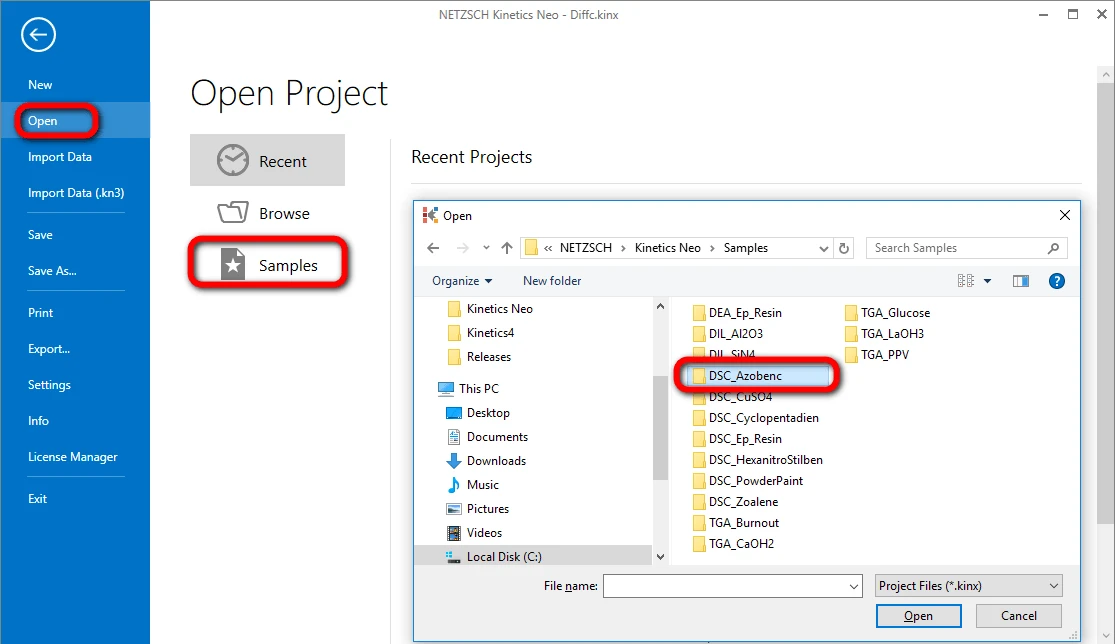
Click on "Open" in the menu on the left side, then select "Samples". The Kinetics Neo samples directory will be opened in Windows Explorer. Select the directory “DSC_Azobenc”.
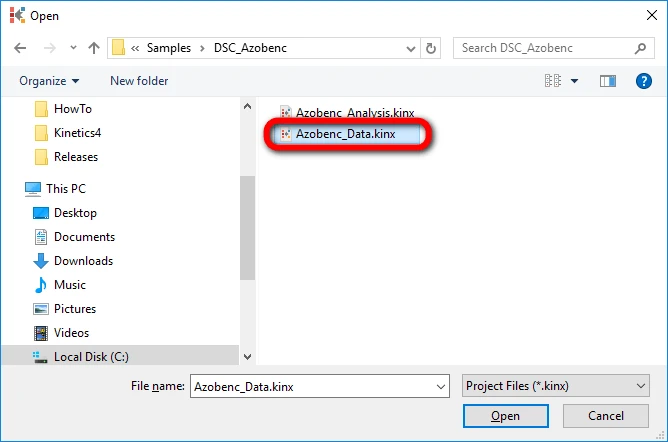
3. Open the Kinetics Neo project file “Azobenc_Data.kinx”.
Check the Loaded Measurement Data
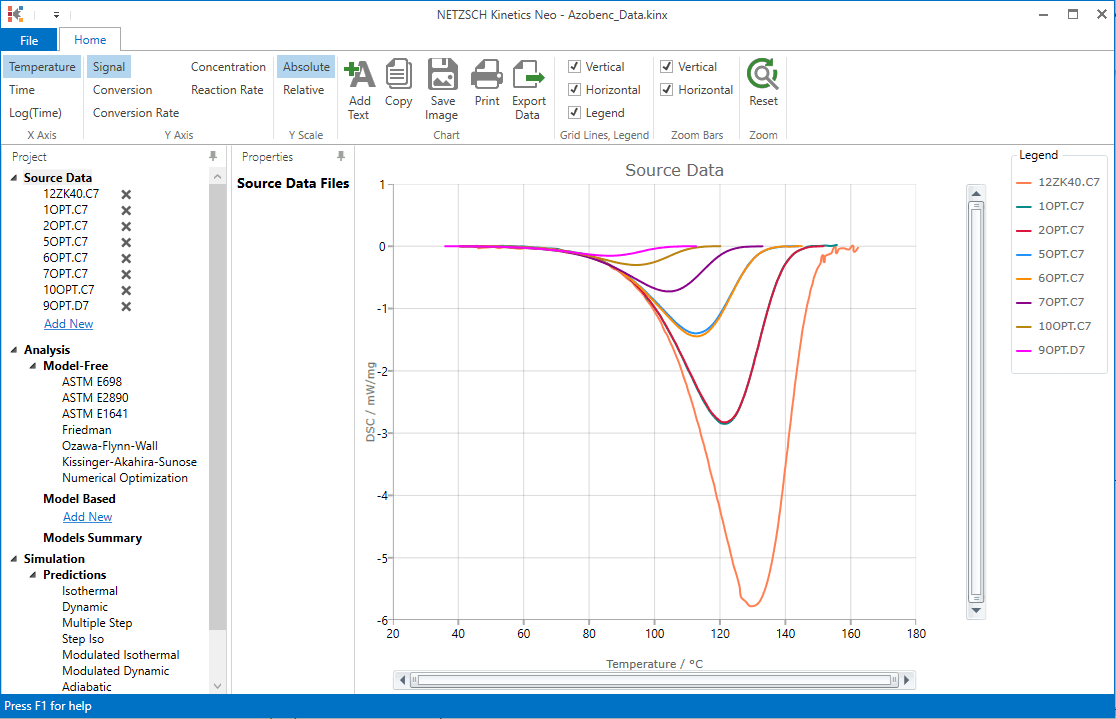
4. Check whether the DSC measurement data are loaded.
The Kinetics Neo sample project "Azobenc_Data.kinx" already contains imported sample DSC measurement data files for sample isomerization:
- 12ZK40.C7- heating rate 40 K/min;
- 1OPT.C7 - heating rate 20 K/min;
- 2OPT.C7- heating rate 20 K/min;
- 5OPT.C7- heating rate 10 K/min;
- 6OPT.C7- heating rate 10 K/min;
- 7OPT.C7- heating rate 5 K/min;
- 1OOpt.C7- heating rate 2 K/min;
- 9OPT.D7- heating rate 1 K/min.
If the project file is successfully loaded then these file names will be seen in the "Source Data" section on the left side. The data curves will be shown on the main chart.
Create a One-Step Kinetic Model (F1)
5. Add new model: In "Analysis" tree under "Model Based" click on "Add New".
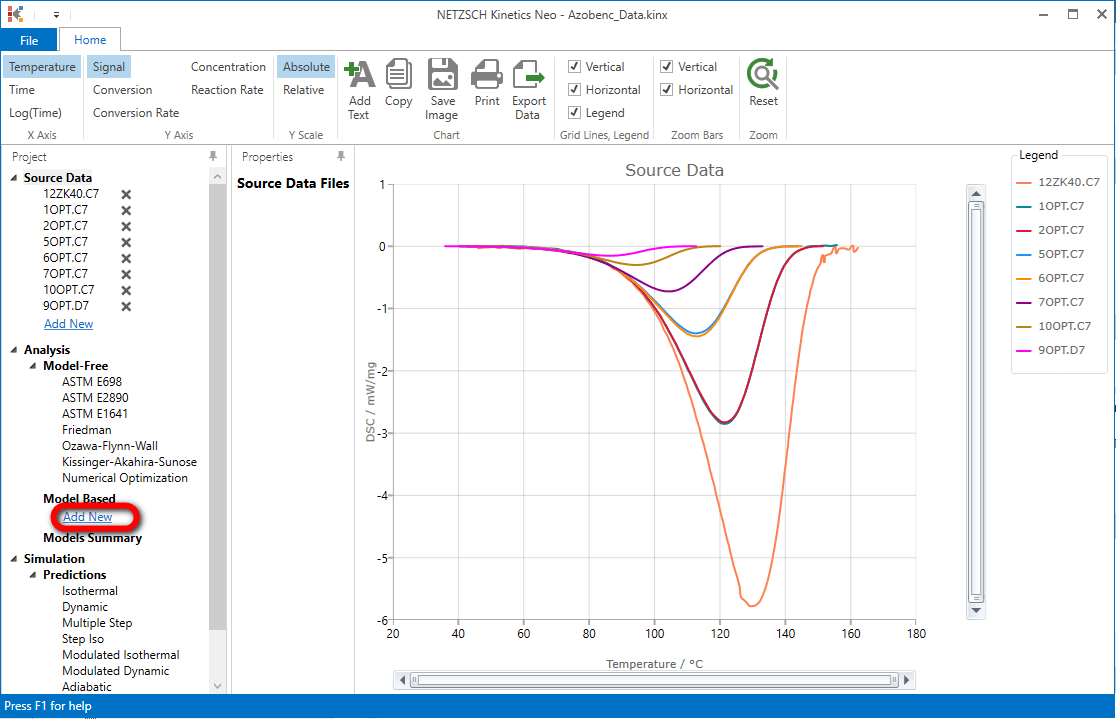
A new "Model Based" kinetic model will be created with default parameters:
- One step: A -> B;
- Reaction type: F1, 1st order reaction.
The first model step A -> B will be selected.
Optionally you can type F1 into the model description text box.
Then, within the Step: A->B section, click on "Optimize" button. The model step parameters: activation energy, pre-exponential factor and contribution will be recalculated and optimized for the this step.
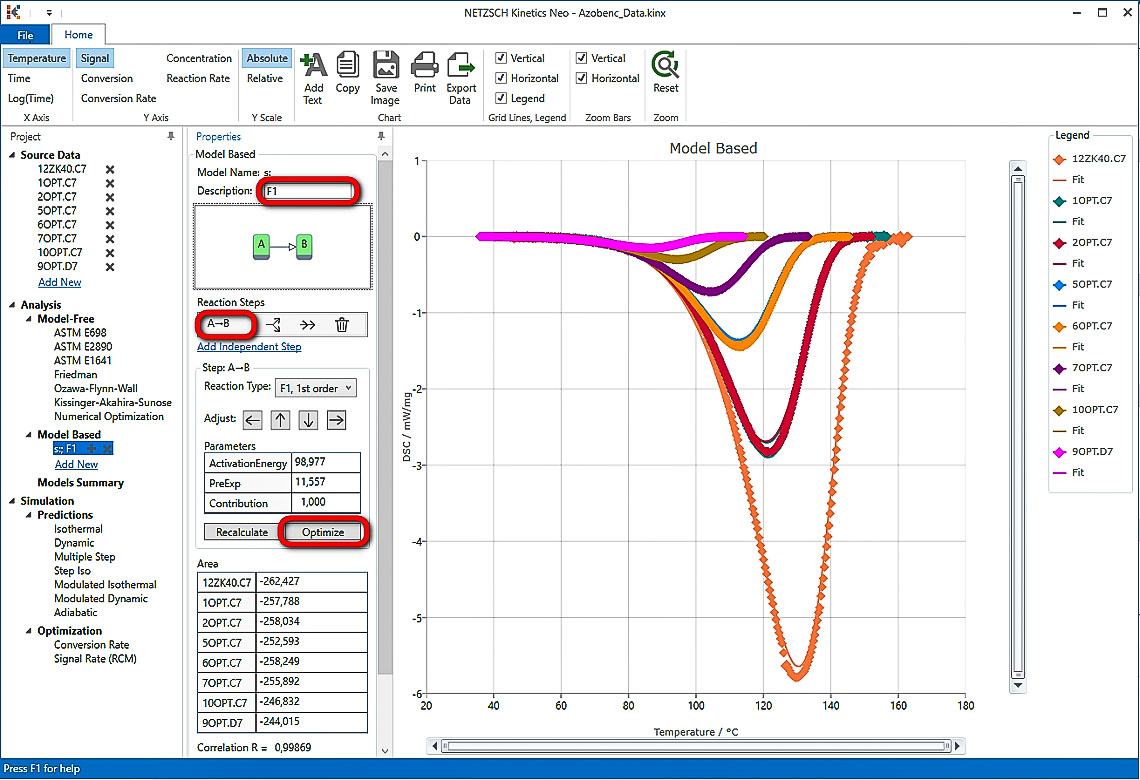
6. Optimize one-step model F1.
In “Model Operations” section at the lower part of Properties panel select “Optimize”. The whole model will be optimized. This may take a few seconds...
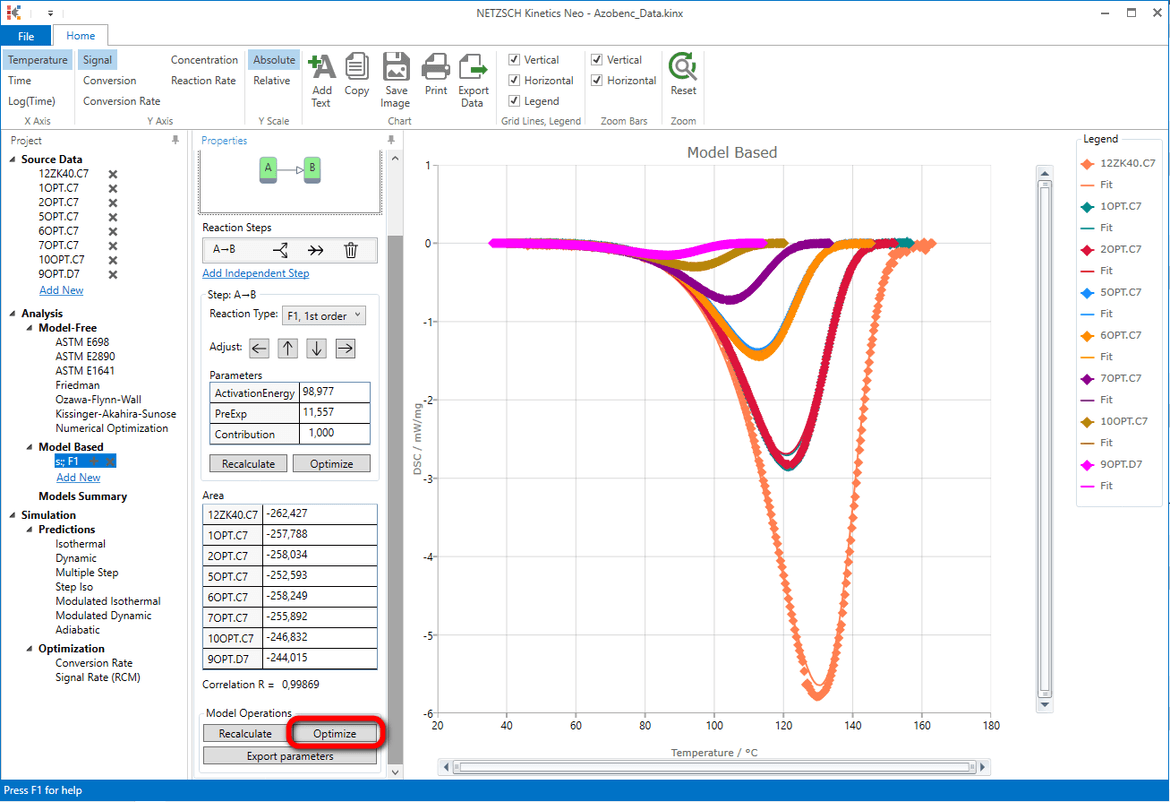
Result after model optimization:
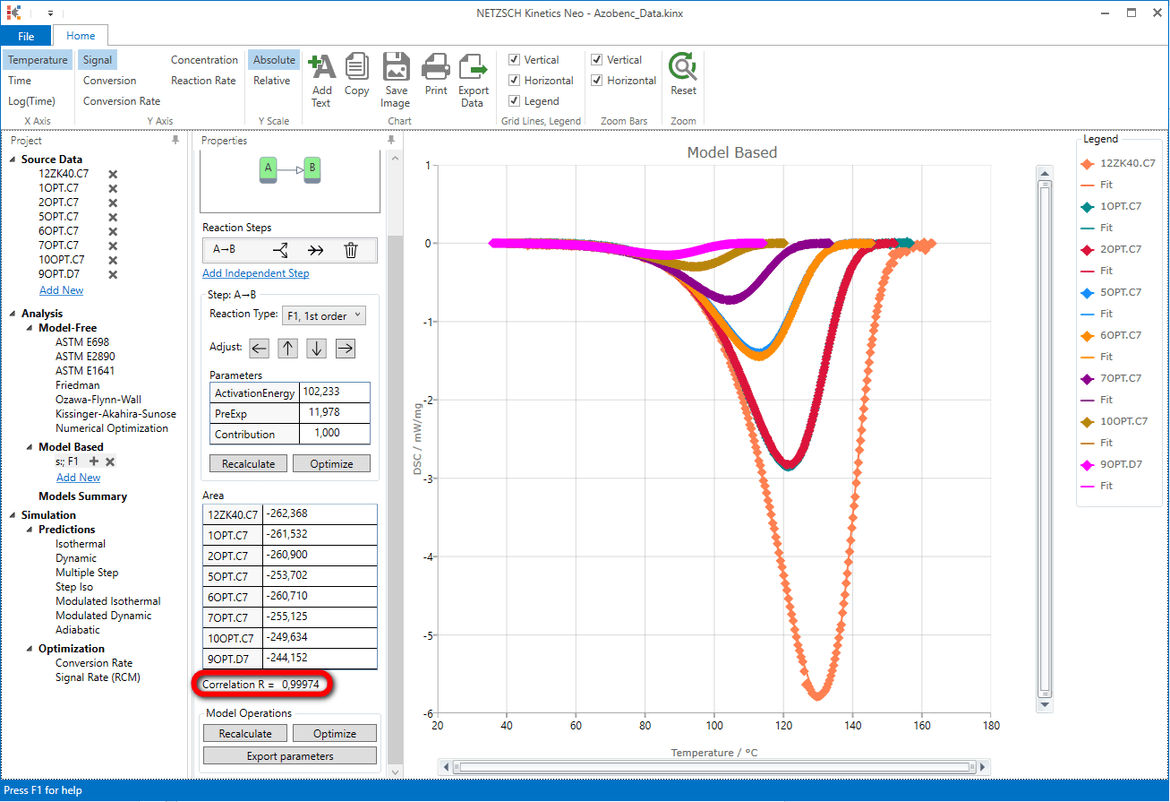
The coefficient of correlation is quite high: R=0.99974 .
Is it good enough?
It is possible to try another model with, for example, a different reaction type and compare the model results with each other. As an example, one may attempt to get a better adaptation of the calculated curves to the measured curves with a reaction type of Fn (n-th order reaction). Then one can assess whether it better fits the source data.
Create a Second One-Step Kinetic Model (Fn). Optional
7. Add new model: In the "Analysis" tree under "Model Based" click on "Add New".
A new "Model Based" kinetic model will be created with the default parameters:
- One step: A->B .
- Reaction type: F1, 1st order reaction.
First reaction step A -> B with reaction type F1 will be selected.
In order to distinguish two models type a "Fn" into the Description text box. in Reaction Type dropbox change the default reaction type F1 to "Fn, n-th order". Then, within the Step: A->B section, click on ”Optimize“ button. The model step parameters: activation energy, pre-exponential factor and contribution will be recalculated and optimized for the this step.
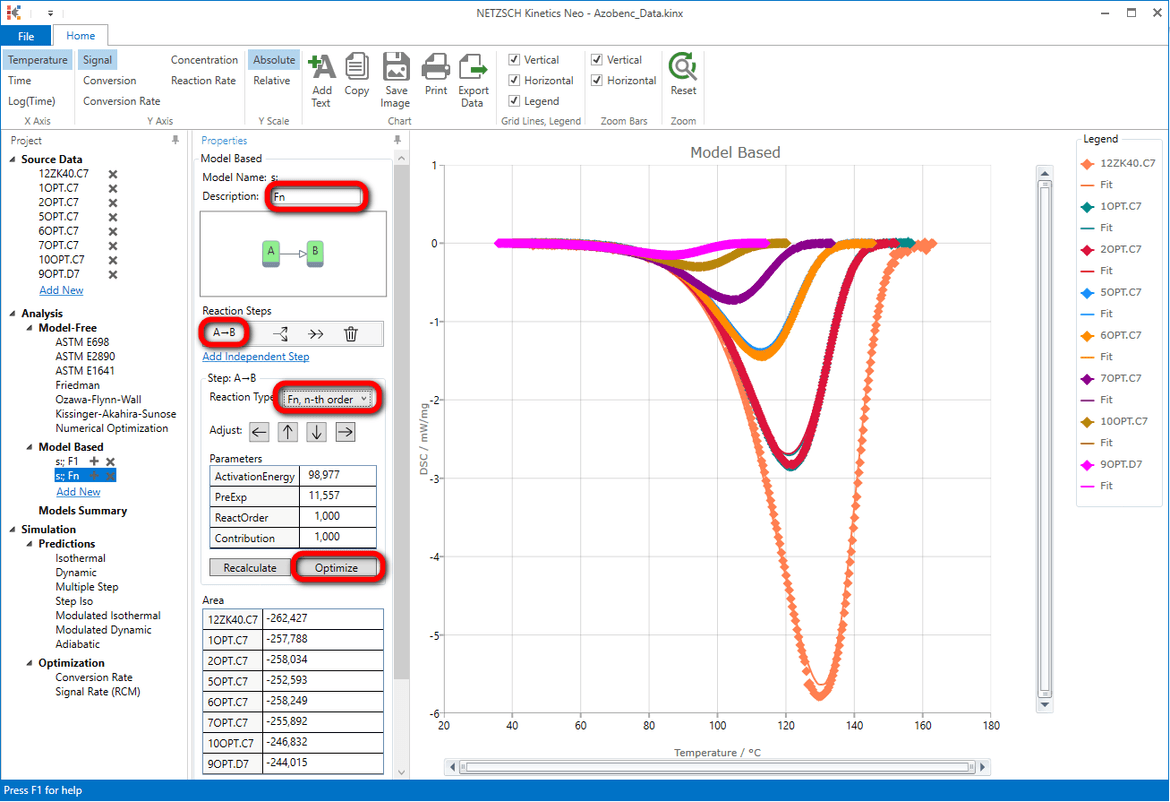
8. Optimize the one-step model Fn.
In the “Model Operations” section in the lower part of the Properties panel select “Optimize”. The whole model will be optimized. This may take a few seconds...
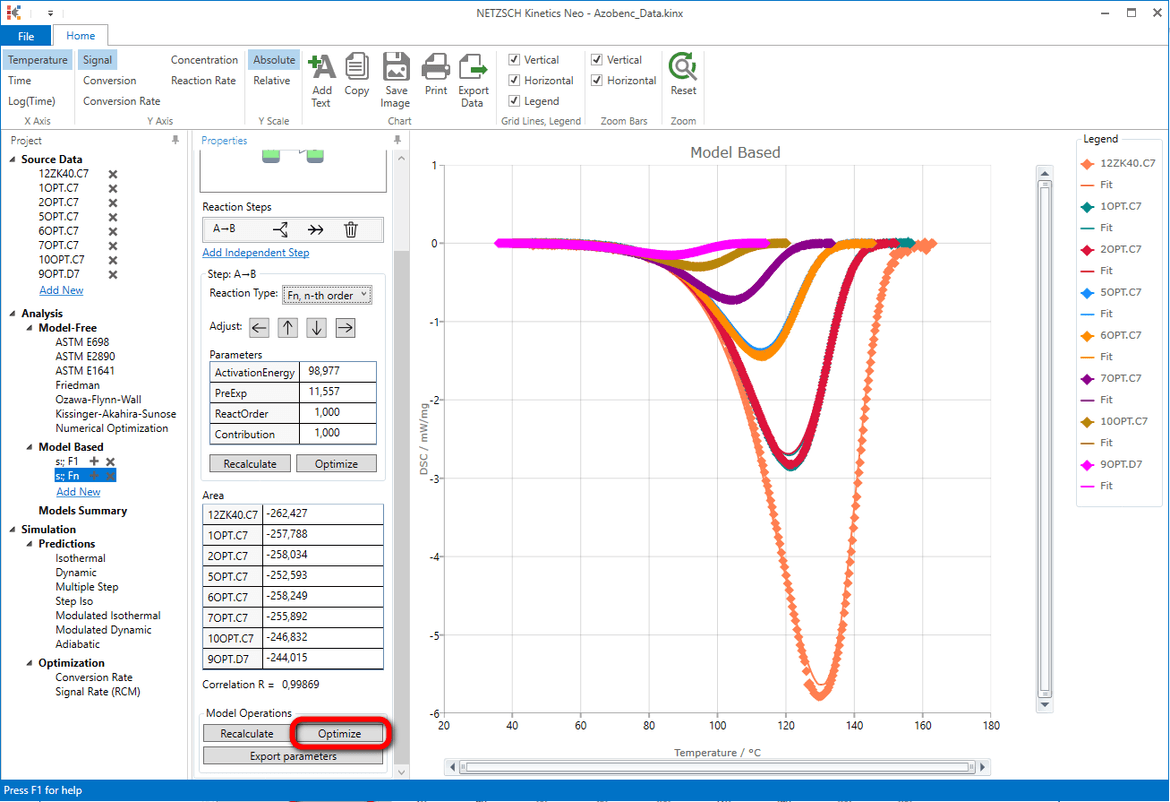
Result after model optimization:
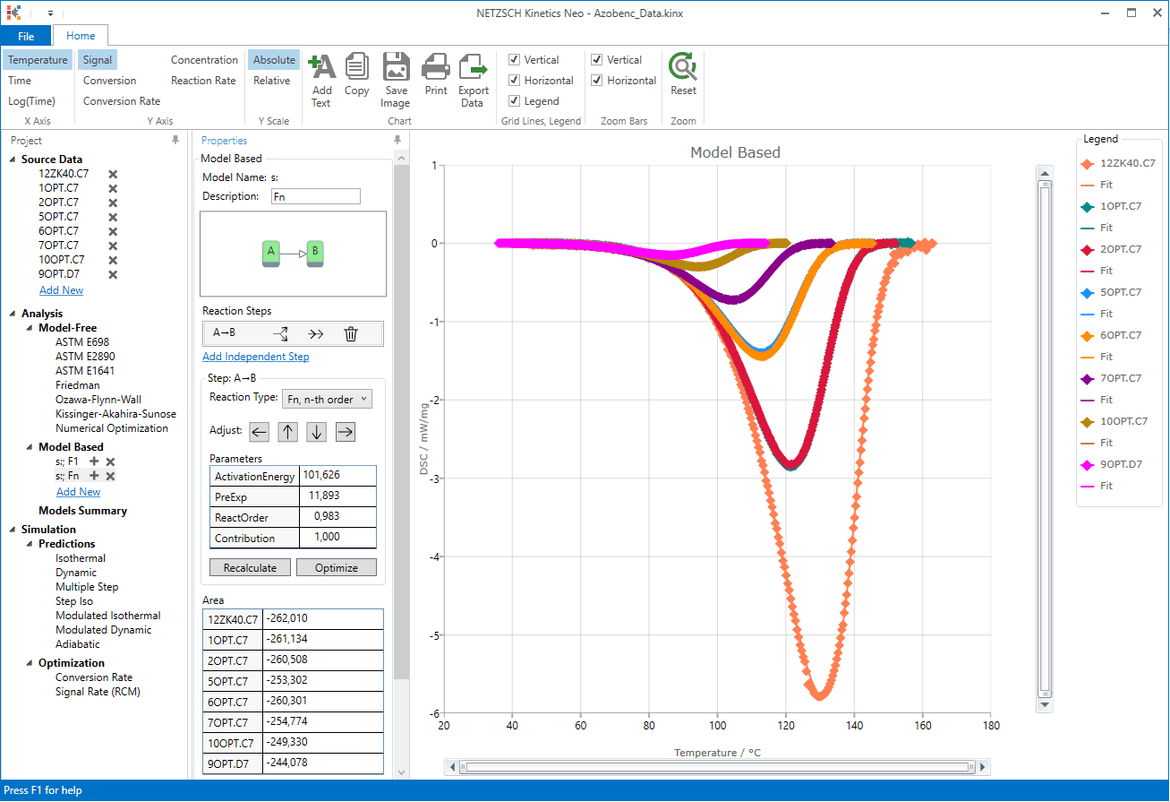
Compare the Two Models. Optional
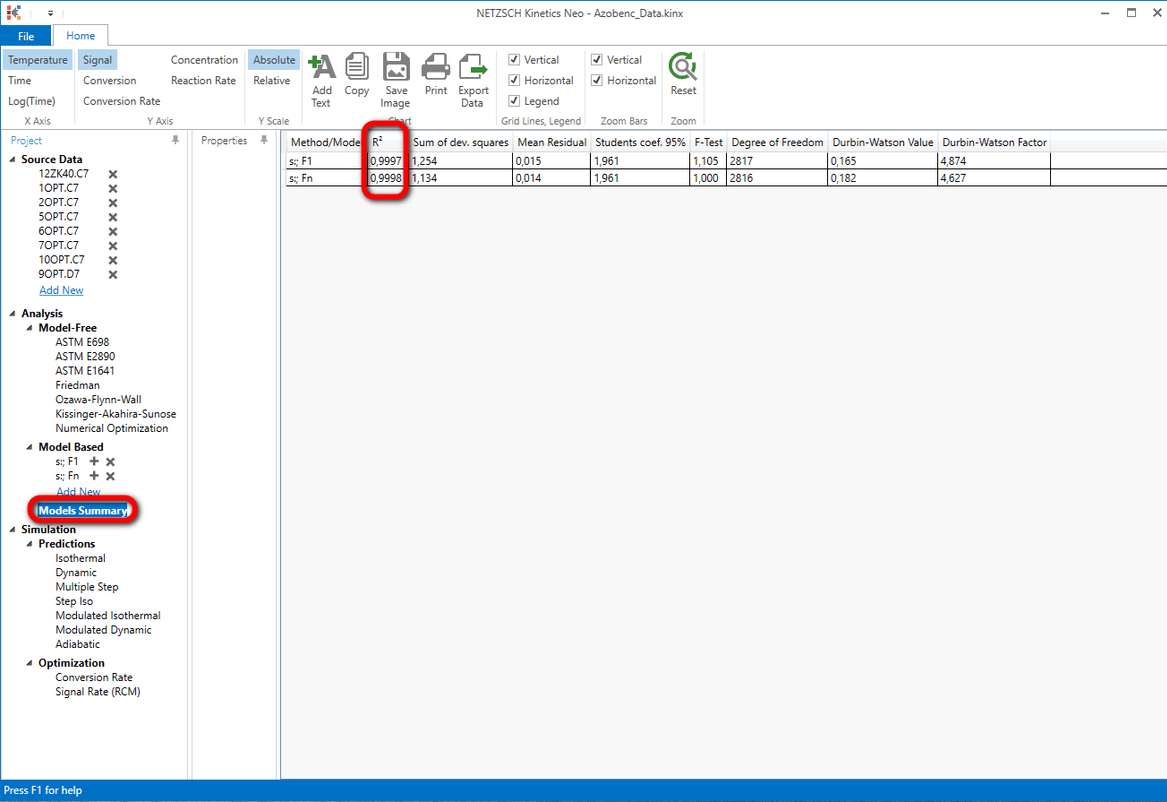
You can compare the statistics of the different calculated models by clicking on "Model Summary" in "Analysis" tree.
A closer look on the coefficients of correlation of both models F1 and Fn shows that they are nearly the same.
Now we have two models. One "s:; F1" contains one reaction step F1 with the first order reaction. The second "s:; Fn" contains also one reaction step, but with reaction of n-th order. The value "n" is more general and can have any numeric value including one.
It is interesting to check which value has the reaction order in the second model.
In "Analysis" tree under "Model Based" select the second model "s:; Fn".
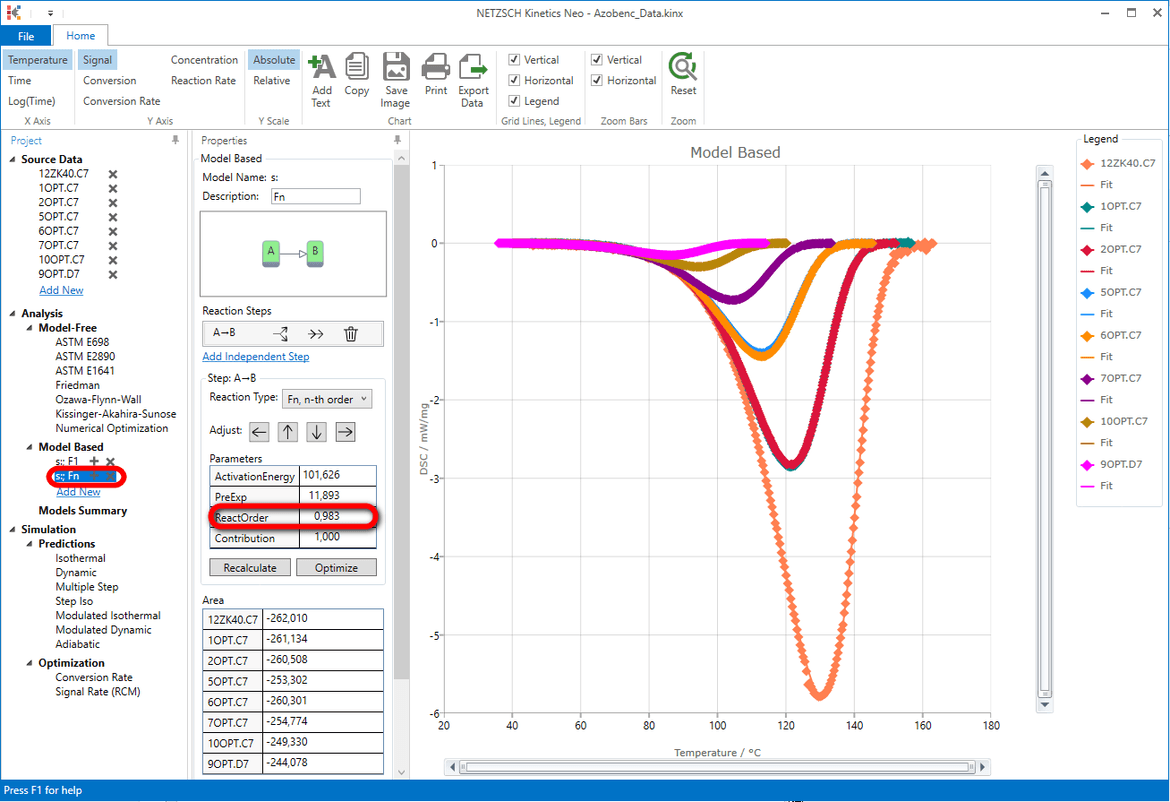
Conclusion
The reaction order of the model "s:; Fn" is nearly 1 (0.982). The model F1 is in fact the model Fn with fixed reaction order 1. This explain why the results of the models F1 and Fn are very similar.
This corresponds to the literature values of the first order reaction for Cis-trans isomerization of subcooled liquid Azobenzene from the following article:
N.Eckhardt, H.J. Flammersheim, H.K.Cammenga. Journal of Thermal Analysis, Vol.52, (1998) 177-185
