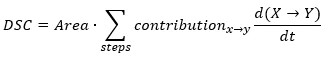What Equations Are Used In My Kinetic Model?
Equations in Kinetics Neo
1. Kinetics model for equations
Let’s have a look at typical example of measurement for three-step reaction. The total mass change is the difference between initial and final mass.
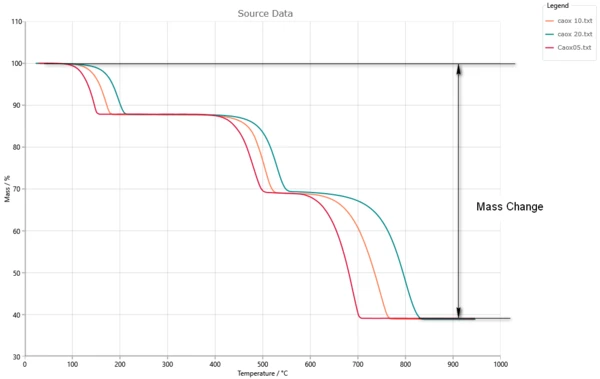
Three-step reaction gives the perfect fit to experiment.
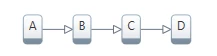
Here A is the initial substance, B and C are intermediate substances, and D is the final product. They have corresponding concentrations a,b,c,d.
2. Export Equations
In order to export model equations to a text file:
- in the tree panel on the left side select the model you are interesting in,
- and in the bottom of Model Based Properties panel click on "Export Parameters" button.
3. Equations for reaction rate for each reaction step
Each reaction step, where reactant X reacts to the product Y, has own parameters and the own equation for its reaction rate.
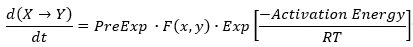
Where:
- R is gas constant,
- T is the absolute Temperature.
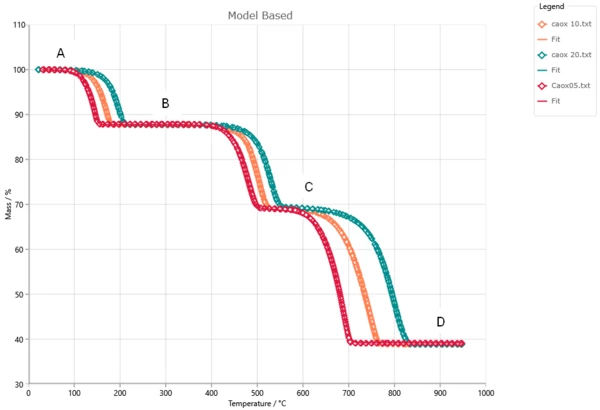
Example of concentrations for all reactants for heating 10K/min. We use the formal concentrations from 0 to 1.
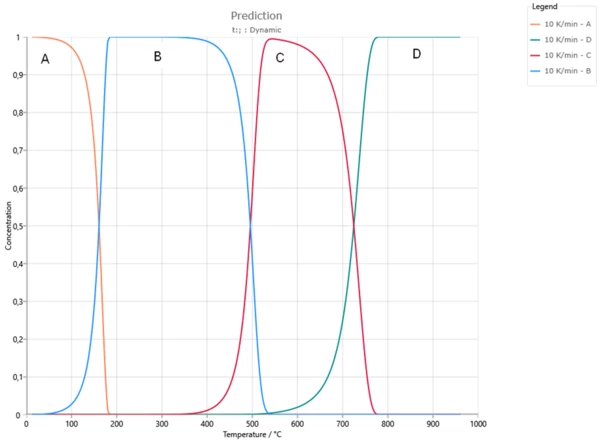
At the beginning the concentration of A is 1, concentrations of B, C and D are zero.
Firstly, A reacts to B. Here concentration of A decreases to zero, and concentration of intermediate product B increases and has maximum at 300°C.
Reaction rate of the first reaction step is:
d(a->b)/dt=PreExp*F(a,b)*Exp[-ActivationEnergy/(RT)]
All individual parameters for this step, like
Activation energy,
- PreExponential factor,
- reaction order,
- reaction type F(a,b)
... are written in the section Step A->B.
Then B reacts to C. Here concentration of B decreases to zero, and concentration of intermediate product C increases and has maximum at 520°C.
Reaction rate of the second reaction step is:
d(b->c)/dt=PreExp*F(b,c)*Exp[-ActivationEnergy/(RT)]
All Parameters for this step are written in the section Step B->C.
Finally C reacts to D. Here concentration of second intermediate product C decreases to zero, and concentration of final product D increases from 0 to 1.
Reaction rate of the second reaction step is:
d(c->d)/dt=PreExp*F(b,c)*Exp[-ActivationEnergy/(RT)]
All Parameters for this step are written in the section Step C->D.
4. Equations for concentrations for each reactant
Each initial reactant has the initial concentration equal to 1 at the begin of the process. This reactant has one or several ways to react and therefore its concentration decreases.
In the current example concentration of initial Reactant A decreases because reaction A->B:
da/dt=-d(a->b)/dt
Each intermediate reactant has the zero concentration the begin of the process. The concentration of intermediate reactant increases because of reaction step where this reactant plays the role of the product. For example, here the concentration of the reactant B increases because of the reaction step A->B.
The concentration of the same intermediate product decreases because of reaction steps where this reactant plays the role of reacting substance. Here the concentration of the reactant B decreases because of the reaction step B->C.
The instant concentration rate for B is the sum of its increasing part and decreasing part:
db/dt=d(a->b)/dt-d(b->c)/dt
Concentration of intermediate reactant C increases because of step B->C and decreases because of step C->D:
dc/dt=d(b->c)/dt-d(c->d)/dt
The initial concentration of final products is zero. It increases because of last reaction step producing this substance. Here the final product D is the result of reaction step C->D and its concentration increases because of this last reaction step.
dd/dt=d(c->d)/dt
At any time point the sum of all concentrations are equal to 1:
a+b+c+d=1
The solving of the system of equations for concentrations and reaction rates allows to find concentrations for each individual reactant in the kinetic model.
5. Balance equations
Balance equations are responsible for the calculation of signal (DSC, TGA,..) from the known reaction rates and concentrations.
5.1 Balance equation for Integral signals
Integral signals are TGA, DIL, DEA, ARC temperature, Rheometry.
For integral data the total signal depends on the sum of the conversions of individual reaction steps.
Each reaction step has the contribution parameter. The contribution shows what part of the total signal change has the current reaction step.
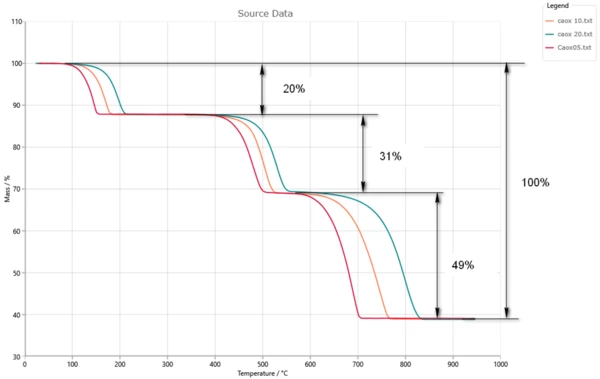
In the picture the total mass loss is 100%, and the contribution of the individual reaction steps are the part of it.
The conversion of each reaction step shows what part of reaction step is already reacted. It is calculated as the integral from the reaction rate for this step from the reaction begin to the current time point:
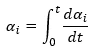
For example, for the first reaction step A->B its conversion is calculated as:
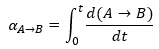
The product of the step conversion and step contribution produces the signal change corresponding to this step. Thus, the sum of these products is part of signal change for all steps, and the signal can be calculated as:
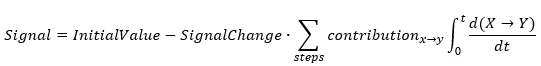
For example, for three-step reaction the exported equation for thermogravimetric signal is written as:
Mass=InitialMass - TotalMasChange*
[Contribution(a->b)*Integral[d(a->b)/dt]dt + Contribution(b->c)*Integral[d(b->c)/dt]dt + Contribution(c->d)*Integral[d(c->d)/dt]dt]
5.2 Balance equation for differential signals
Differential signals are DSC, MS.
For differential data the total signal depends on the sum of the reaction rates of individual reaction steps.
Each reaction step has the contribution parameter. The contribution shows what part of the total peak area has the current reaction step. For DSC the total signal is calculated as the following expression: