Model-Free Kinetic Analysis of Poplar Fluff (Populus Alba) Pyrolysis Process under Dynamic (Non-Isothermal) Conditions
Nebojša Manić, Bojan Janković. University of Belgrade, Serbia.
Keywords
Pyrolysis, biofuel, biomass, thermogravimetry, STA, Kinetics Neo, model free methods, isoconversional methods, poplar fluff.
Sample
As representative among biomass feedstocks, Poplar fluff was chosen to analyze the pyrolysis properties of this new biofuel. Poplar fluff is sterile seeds embedded in a dispersal mechanism of fluffy material. This fluff is considered as precious natural material (fibers) with unique morphological structure applicable in modern technology (production of natural super sorbents or smart medical materials). Fibrous seeds can be treated as lignocellulosic waste material.
Experimental Tests
Thermal analysis apparatus: NETZSCH STA 445 Jupiter F5 System with alumina crucibles:
- High-purity argon , gas flow 30mL/min, protective gas flow 20mL/min
- Sample masses are about Δm = 5.0 ± 0.3 mg
- Sample was heated from the room temperature (RT) up to 800 °C with heating rates 5,10,15 and 20K/min.
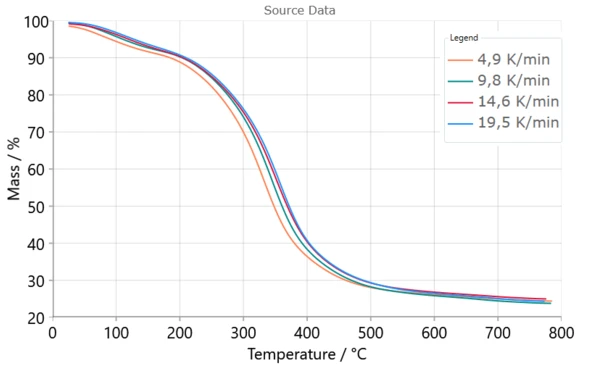
Results and Discussion
Friedman, Ozawa–Flynn–Wall (OFW) and Kissinger–Akahira–Sunose (KAS) Model-Free Methods
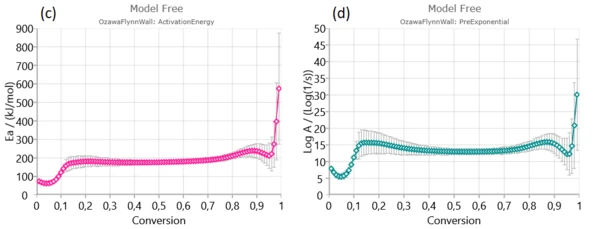
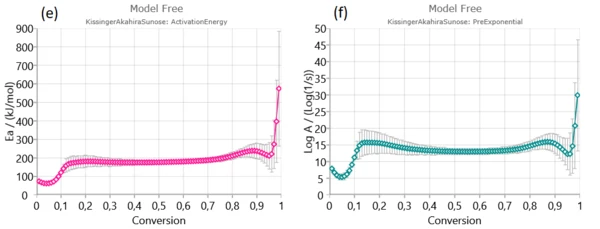
Firstly, Friedman (a), Ozawa–Flynn–Wall (OFW) (b) and Kissinger–Akahira–Sunose (KAS) were applied to experimental data in order to find activation energy and pre-exponential factor for each degree of conversion.
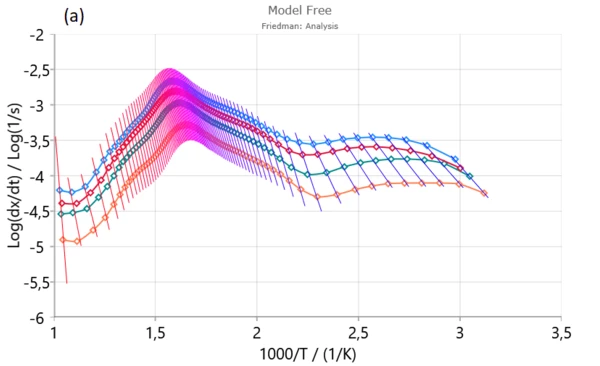
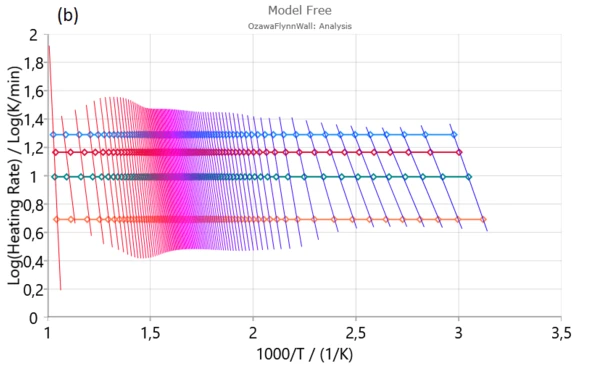
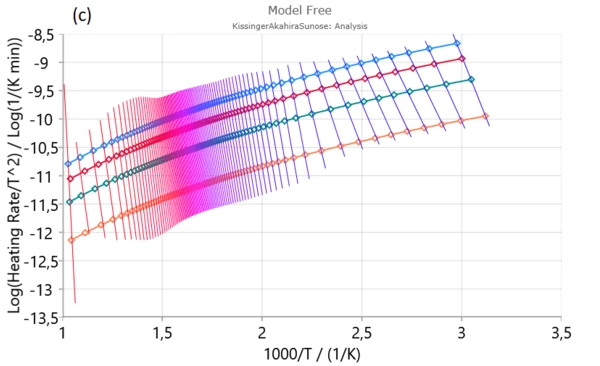
From isoconversional plots (Figure2), it can be observed that in considered conversion levels range, the isoconversional lines are not parallel and change their slope with the process progress. This indicates the high probability of changing kinetic parameters during pyrolysis process. However, at the medium conversion levels which belong primarily to devolatilization stage, isoconversional lines are almost parallel and kinetic parameters in the indicated region can be stable without a significant variation – Figure 1. [1].
In conversion region with high density of parallel isoconversional lines , we can expect that the process takes place via single-step reaction mechanism. In the entire pyrolysis process, the initial (up to α ~ 0.13) and final (above α ~ 0.90) stages were characterized by a change in the slope of regression lines and this is a consequence of the occurrence of multiple reactions. Such changes are less noticeable in the case of integral model-free methods (such as OFW and KAS) In this sense, the Friedman’s method is much more flexible.
Based on Friedman analysis, the Friedman isoconversional plots create three characteristic reactivity surfaces (Figure 2 (a)).
Plots of activation energy and pre-exponential factor present three decomposition steps:
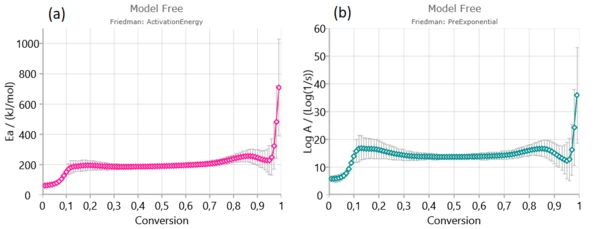
The first stage which is related to elimination of physical water, ascribed as the moisture content, is followed by increasing in activation energy value in the range of ~ 62 kJ/mol (Friedman method) to approximately 190 kJ/mol for conversion up to α ~ 0.13/0.14.
Next stage is mainly attributed to decomposition of cellulose and in-part of hemicelluloses decomposition.It is characterized by stable magnitudes of both kinetic parameters (Ea and log A) (Figure 3). Estimated mean values of activation energies as 190.574 kJ/mol – Friedman and 177.787 kJ/mol – OFW, and 177.702 kJ/mol – KAS, respectively, are very similar to the activation energy values related for the most fibers: 156–175 kJ/mol [1].
After α = 0.65, it can be observed increased behavior of both kinetic parameter’s values (Ea and log A) with an increasing of α. This last stage belongs to the decomposition of majority lignin molecule. In addition, after α ~ 0.95, the activation energy suddenly increases because of the increase in the thermal stability as a result of the increasing aromatic character of the lignin- derived bio-char at higher temperatures. Increasing trend of Ea values at high conversion at α > 0.80 was observed for extracted materials, such as holocellulose and α-cellulose, and can be attributed to the formation of the aromatic polycyclic structure of the higher thermal stability [1].
Numerical Optimization Model-Free Method
The next iso-conversional method is Numerical optimization of pyrolysis process.
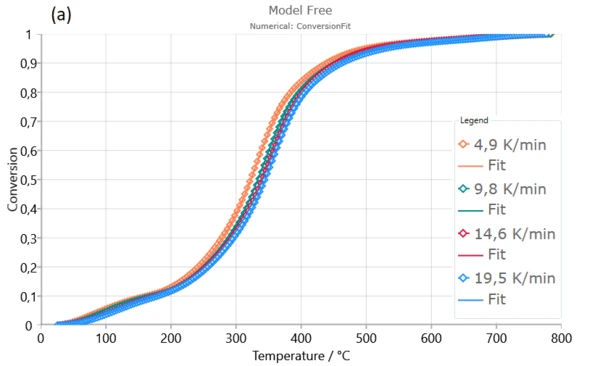
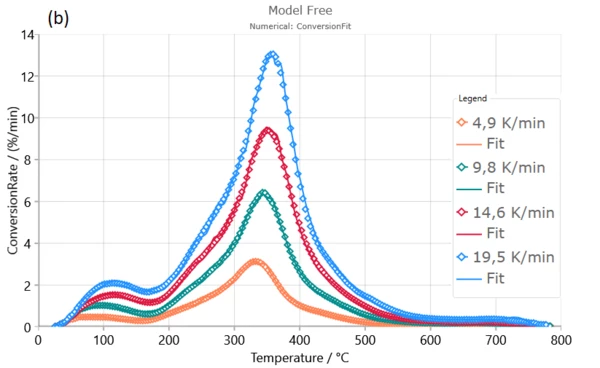
Numerical optimization model-free model gives excellent fits of the experimental data and very well describes the entire pyrolysis process, including all reaction stages. The correlation quantity ( R ) is extremely high in both cases which confirm that calculated kinetic parameters from model-free model are true and physically justified for dynamic pyrolysis process of Poplar fluff [1].
References
[1] Nebojša Manić, Bojan Janković & Vladimir Dodevski, Model-free and model-based kinetic analysis of Poplar fluff (Populus alba) pyrolysis process under dynamic conditions. J. Therm. Anal. Calorim. (2020). doi.org/10.1007/s10973-020-09675-y.
This document was prepared by Prof. Nebojša Manić 1 and Dr. Bojan Janković 2.
Adapted to Application Note by Dr. Elena Moukhina, NETZSCH.
1 University of Belgrade, Fuel and Combustion Laboratory, Faculty of Mechanical Engineering, Kraljice Marije 16, P.O. Box 35, 11120 Belgrade, Serbia.
2 University of Belgrade, “Vinča” Institute of Nuclear Sciences - National Institute of thе Republic of Serbia, Department of Physical Chemistry, Mike Petrovića Alasa 12-14, P.O. Box 522, 11001 Belgrade, Serbia.
