Model-Based Analysis of Poplar Fluff (Populus Alba) Pyrolysis Process under Dynamic (Non-Isothermal) Conditions
Nebojša Manić, Bojan Janković. University of Belgrade, Serbia.
Keywords
Pyrolysis, biofuel, biomass, thermogravimetry, STA, Kinetics Neo, model free methods, isoconversional methods, poplar fluff.
Sample
As representative among biomass feedstocks, Poplar fluff was chosen to analyze the pyrolysis properties of this new biofuel. Poplar fluff is sterile seeds embedded in a dispersal mechanism of fluffy material . This fluff is considered as precious natural material (fibers) with unique morphological structure applicable in modern technology (production of natural super sorbents or smart medical materials). Fibrous seeds can be treated as lignocellulosic waste material.
Experimental Tests
Thermal analysis apparatus: NETZSCH STA 445 Jupiter F5 System with alumina crucibles:
- High-purity argon , gas flow 30mL/min, protective gas flow 20mL/min
- Sample masses are about Δm = 5.0 ± 0.3 mg
- Sample was heated from the room temperature (RT) up to 800 °C with heating rates 5,10,15 and 20K/min.
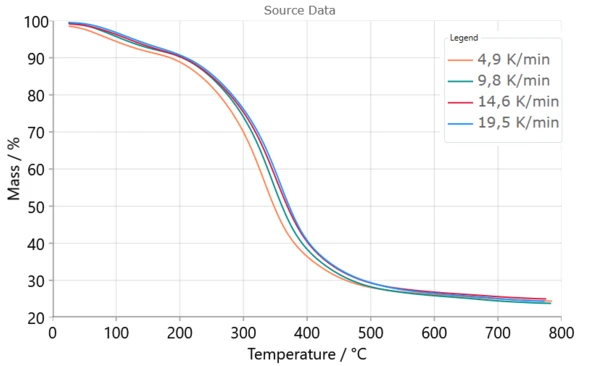
Advances of Model-Based Approach in Respect to Model-Free Methods
Model-based approach provides information about the number of reaction steps that occur during pyrolysis process, as a global mechanistic scheme taking into account derived kinetic parameters and contribution of each step to overall process. In comparison with model-free approaches, which do not give any information about features of a reaction mechanism, the model-based computational approach gives correct prediction of the course of the complex reaction.
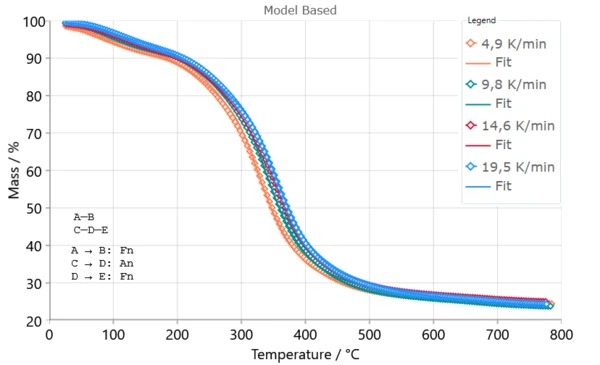
Three-step mechanism (A → B, C → D → E) represents the topgallant accepted mechanism as the most probable for the pyrolysis process of Poplar fluff, where each step is described by either n-dimensional nucleation Avrami–Erofeev model (Code An : n is Avrami kinetic exponent) [1] or reaction of n-th order (Code Fn).
| Step Parameters | Fn (A → B) | An (C → D) | Fn (D → E) |
|---|---|---|---|
| Ea /(kJ/mol) | 74.061 | 236.190 | 99.578 |
| log A (1/s) | 8.987 | 19.457 | 6.903 |
| n | 7.716 | 0.186 | 4.933 |
| Contribution | 0.115 | 0.363 | 0.522 |
The kinetic model of An reaction type represents most suitable model for describing the pyrolysis process through auto-acceleratory model such as Avrami–Erofeev because this model was proposed for the description of nucleation driven process, where the growth rate is significantly reduces because of intensified fragmentation and emission of volatiles [1]. For all steps the dimension of reaction is less than unity (n < 1), and its variation through the steps is connected with changes in Ea values through multiple elemental steps [1].
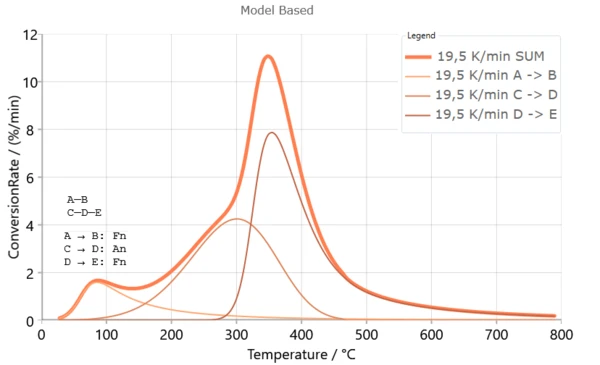
Reaction Mechanism for Poplar Fluff Fiber Pyrolysis
Reaction mechanism for Poplar fluff fiber pyrolysis can be summarized as follows:
- the component A is hemicelluloses and cellulose,
- the component C consists of volatile products, biochar residue from thermal decomposition of hemicelluloses and “inactive” form of the cellulose, and
- the component D represents “active” form of the cellulose, and finally
- the component E was formed by the volatile products, the residual ash and pyrolyzate tar that results from the thermal decomposition of cellulose at the higher temperatures [1].
Since the biomass is carbonaceous solid, upon the heating its porous network develops and the surface area increases. As the solid devolatilizes (step A → B), the gaseous products that form must diffuse to the surface of the particles. This diffusion can limit the reaction rate. So, diffusion-limited process may be one of the possible approximations which can be included in kinetics investigation of the pyrolysis process [1].
First Step (A → B)
The first stage (step A→B) belongs to simultaneously occurring of moisture evaporation and starting decomposition reactions linked with hemicelluloses and lignin; During this step with low value of activation energy, the gas phase polymerization of volatiles to refractory condensable (tar) product may appear (~ 60 - 70 kJ/mol), which is in accordance with solid-phase pyrolysis chemistry [1].
Second Step (consecutive step C → D)
The second step (consequitive step C → D) in the temperature range of 180–400 °C belongs to cellulose pyrolysis and this stage can be characterized by a set of concurrent and consecutive reactions This transition step from “inactive” to “active” form of the cellulose and released volatile products has the greatest contribution among all considered steps in entire pyrolysis process, which is in fully agreement with previously presented results and settled assumptions. Ea value of 236 kJ/mol for this step (C → D) is within a range of Ea value for mechanism with gas phase cracking of volatiles to permanent gas species (200-300 kJ mol-1) from levoglucosan (step D → E).
Third Step (D → E)
Finally (step D → E), beyond 400 °C, the bio-char formation is lasting (ash and carbon are left as the remaining compositions slowly) [1]. However, above 400 °C, rapid depolymerization of cellulose molecules can proceed, largely by cleavage of glycosyl units leading to formation of 1,6- anhydro-β-dglucopyranose (levoglucosan) (see [1]).
Examples of Poplar Fluff Pyrolysis Predictions for Modulated and Multiple-Step Dynamic Conditions
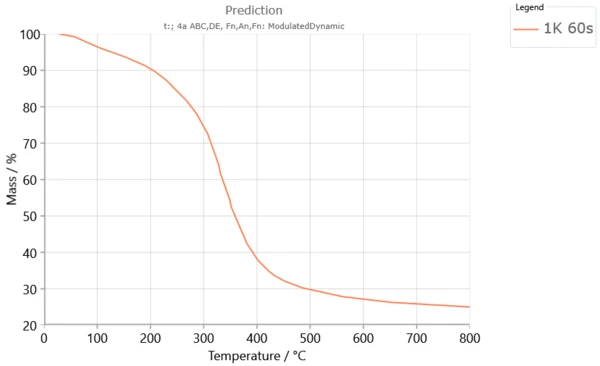
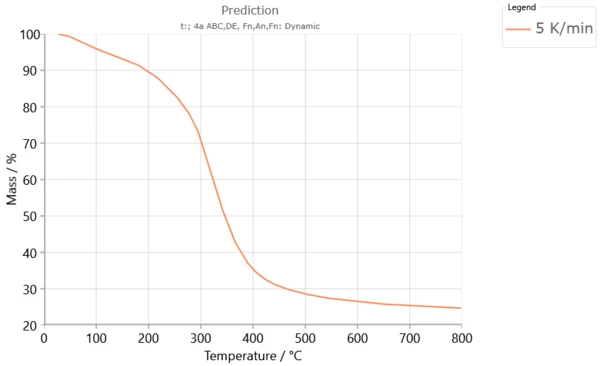
Selected conditions for construction of modulated and predicted multiple-step TG curves of the Poplar fluff pyrolysis process in accordance with An model, performed by the software Kinetics Neo are given in Table 1.
Table 1. Conditions for construction of modulated and predicted multiple-step TG curves
| Modulated dynamic TG Set parameters | |
|---|---|
| Min., T / oC | 25 |
| Max., T / oC | 800 |
| Heating Rate, β / (K/min) | 10 |
| Period, t / s | 60 |
| Amplitude / K | 1 |
| Predicted multiple-step TG Set parameters | |
|---|---|
| Start T / oC | 25 |
| End T / oC | 800 |
| Heating Rate, β / (K/min) | 5 |
| Time, t / min | 150 |
Shapes of modulated and multiple-step TG curves (Figures 4 - 5) are similar to each other that exhibit similar thermal stability. Three distinct mass loss stages could be determined and it is in agreement with each other. Since that present multiple step dynamic TG curve of pyrolysis process was conducted at low heating rate values, the largest fraction of biomass constituent is decomposed below 400 °C. Chemical reactions under modulated heating program could include reduction of depolymerization by bond scission, appearance of free radicals, elimination of H2O, and formation of carbonyl and carboxyl groups, evolution of CO and CO2 gases, and finally production of charred residue.
Reference
[1] Nebojša Manić, Bojan Janković & Vladimir Dodevski, Model-free and model-based kinetic analysis of Poplar fluff (Populus alba) pyrolysis process under dynamic conditions. J. Therm. Anal. Calorim. (2020). doi.org/10.1007/s10973-020-09675-y.
Document was prepared by Prof. Nebojša Manić1 and Dr Bojan Janković2.
Adapted to Application Note by Dr. Elena Moukhina, NETZSCH.
1 University of Belgrade, Fuel and Combustion Laboratory, Faculty of Mechanical Engineering, Kraljice Marije 16, P.O. Box 35, 11120 Belgrade, Serbia.
2 University of Belgrade, Department of Physical Chemistry, “Vinča” Institute of Nuclear Sciences - National Institute of thе Republic of Serbia, Mike Petrovića Alasa 12-14, P.O. Box 522, 11001 Belgrade, Serbia.
