How To: Create a Simple Single Step Kinetic Model for TGA Data
Thermal Decomposition of Calcium Hydroxide
Introduction
In this "How To:", a simple kinetic model will be created.
We will start by loading a sample data project included in Kinetics Neo, will then create a simple kinetics model consisting of only one step, and finally will optimize it.
Just a few clicks in over the course of a few minutes - and you will have your first kinetics model!
Sample data:
- Data Type: Thermogravimetry (TGA)
- Project File: CaOH2.kinx
Load the Sample Data Project
1. Start the Kinetics Neo software. Click on the blue "File" tab to open the application menu.
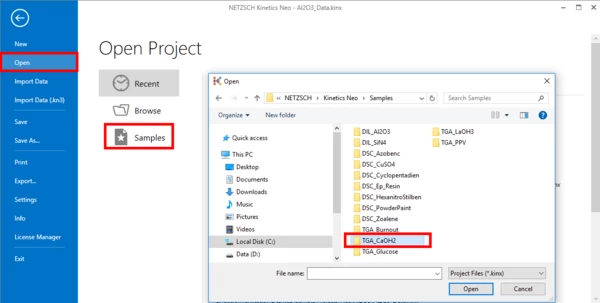
2. Click on "Open" in the menu on the left side, then select "Samples".
The Kinetics Neo samples directory will be opened in Windows Explorer.
Select directory "TGA_CaOH2".
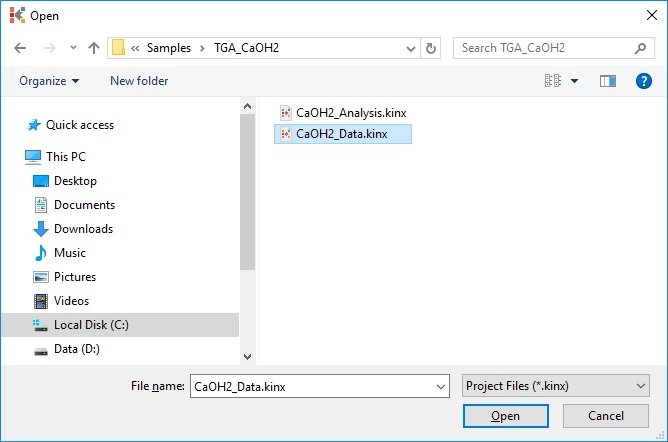
3. Open the Kinetics Neo project file "CaOH2_Data.kinx" .
Check the Loaded Measurement Data
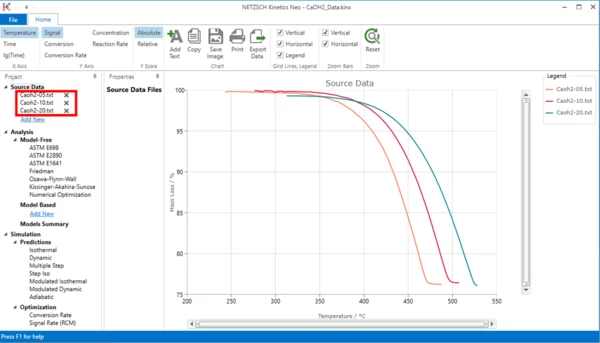
4. Check whether the TGA measurement data are loaded.
The Kinetics Neo sample project "CaOH2_Data.kinx" already contains imported sample thermogravimetrical (TGA) measurement data files for Calcium Hydroxide decomposition:
- CaOH2-05.txt - heating rate 5 K/min;
- CaOH2-10.txt - heating rate 10 K/min;
- CaOH2-20.txt - heating rate 20 K/min.
If the project file is successfully loaded then these file names will be seen in the "Source Data" section on the left side. The data curves will be shown on the main chart.
Create a Simple Kinetic Model
5. In "Analysis" tree, under "Model Based" item click on "Add New".
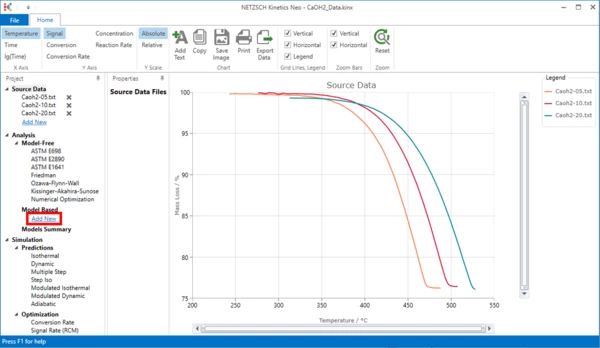
6. A new "Model Based" kinetic model will be created.
This new model has the following default parameters:
- One step: A -> B.
- Reaction Type: F1, 1st order.
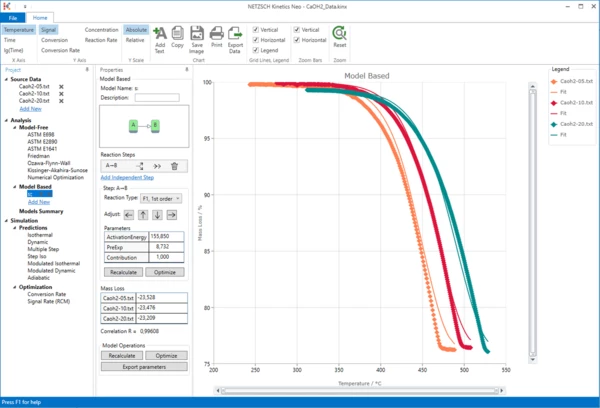
Change the Kinetic Model
7. Let's change some parameters of the model.
We are not sure that this reaction has the reaction order "1". In this case, it is recommended to select the general reaction order "n", which would also include the value of "1". Using model optimization, the software will determine the correct reaction order on its own.
First of all, in the Reaction Type drop menu change the reaction type from the default value "F1, 1st order" to "Fn, n-th order" . Then, within the Step: A->B section, click on "Optimize" button.
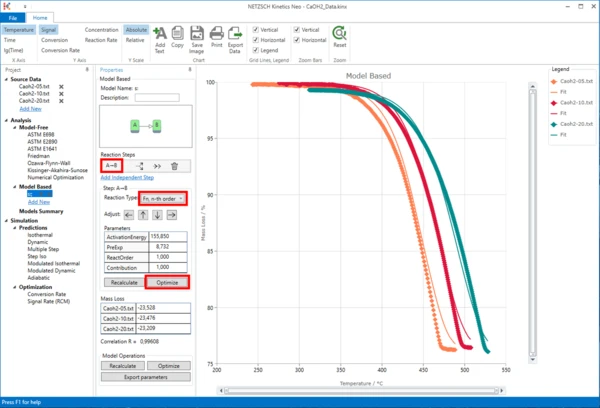
The model step will be optimized. This may take a few seconds...
Model Optimization
8. The results are already quite good. But in some places, especially at the ends of the curves, the simulated data do not perfectly fit the measurement.
Let's optimize the model. At the bottom of the Properties column- in the "Model Operations" section, click on the "Optimize" button:
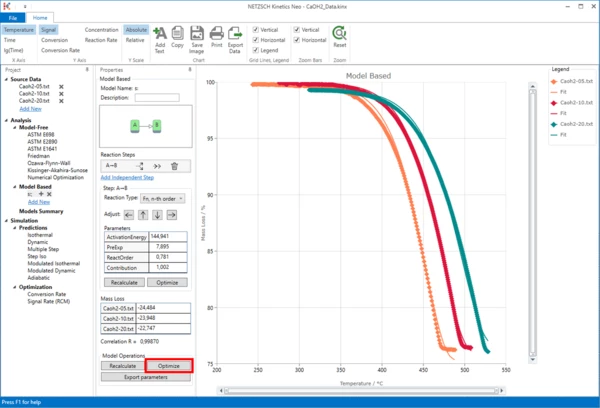
Results
9. Now the simulated data are in good agreement with the experiment.
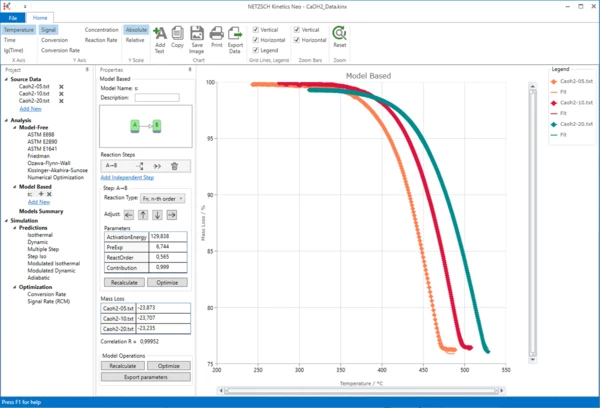
Conclusion
The degradation of Ca(OH)2 can be described as a one-step reaction with reaction order 0.5 and without autocatalysis.
